What disease components are most burdensome to patients? How do researchers find this information out?
To answer this question, the Food and Drug Administration (FDA) recently put out its second guidance document on Patient-Focused Drug Development: Methods to Identify What Is Important to Patients. A few qualitative approaches they recommend to collect this information include:
One-on-one interviews offer the opportunity to explore topics in-depth at an individual level using probing questions.
- Approaches: There are three different types of interview methods: structured interviews (interviewer asks a set of predefined questions), semi-structured interviews (interviewer asks a set of predefined questions and probing questions), unstructured interviews (interviewer asks unplanned or spontaneous questions).
- Patient population: Patients recruited for these interviews should be broadly representative of the patient population of interest and a sufficient sample size should be collect to capture the breadth of opinions on the disease.
- Administration: Interviews approach should be pilot tested before a full roll-out begins. Interviewers should consider the pros and cons of remote vs. in-person interviews. Interviewers should be experienced and or well-trained. Interviewers should be cognizant of the emotional burden the questions could place on the respondent.
- Strengths: Interviews are useful for obtaining more in-depth individual experiences; addressing sensitive topics; and exploring diseases or conditions with many different symptoms that vary from patient to patient
- Limitations.
Focus groups involve a conversation with a group of participants led by a moderator. The most common type is the single focus group, an interactive discussion of a topic by a set of participants and a moderator(s) as one group in one place. Generally, a moderator uses a semi-structured discussion guide to direct the group conversation.
- Number of focus groups. This may depend on (i) the complexity of the topic (e.g., all versus some impacts of a disease or condition on multiple dimensions of a patient’s quality of life, dependent upon therapeutic area and research question, (ii) topic sensitivity (e.g., separation of genders for sensitive topics such as sexual function, (iii) diversity of participant sample, (iv) number of subgroups planned.
- Size of focus group. The goal is make the group big enough to allow for sufficient diversity of opinion while keeping it small enough so all participants can weigh in. Samples sizes of 5 to 10 participants per focus group is most common.
- Moderator. Like in the case of interviews, the moderator (or interviewer) should be well-trained with expertise in the subject area.
- Strengths: Allow for participant interaction; permits capture of a range of perspectives within a shorter time frame (compared to one-on-one interviews).
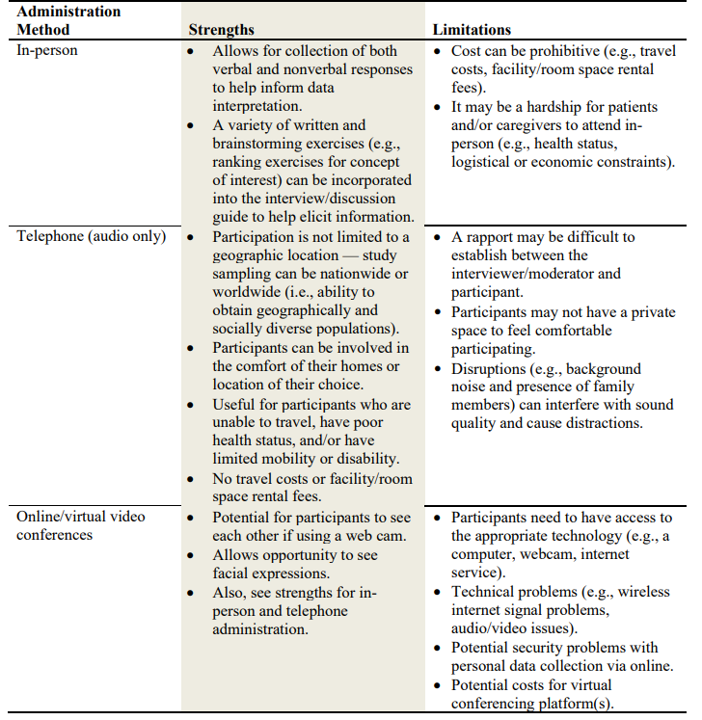
One must also decide whether the qualitative research should be done in-person, on the telephone, or via video conference. Some of the pros and cons of each approach are outlined in the table below.
The FDA guidance also provides some (high-level) recommendations on both quantitative surveys and mixed methods approaches, as well as discussion on the use of social media for both passive and active data collection.